Theme: New Tides in Pharma Industry
Pharmaceutical Chemistry 2020
ConferenceSeries LLC Ltd is a renowned organization that organizes highly notable pharmaceutical, Chemistry and other Life Sciences conferences throughout the globe. ConferenceSeries LLC Ltd organizes 600+ Conferences every year across USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contain over 70000 eminent personalities, reputed scientists as editorial board members, 1200 Symposium & Workshops and 5 million followers.
ConferenceSeries LLC Ltd invites all the participants from all over the world to attend ‘15th World Conference on Pharmaceutical Chemistry’ during November 23-24, 2020 in London, Uk which includes prompt keynote presentations, Oral talks, Poster presentations, Workshops and Exhibitions.
Pharmaceutical Chemistry 2020 is a global overview the Theme: “New Tides in Pharma Industry”. It is a pavement for Pharmaceutical and Chemistry experts and scientific professionals to explore the research challenges & current trends and enleash advanced strategies in the arena of Pharma and Chemistry research areas. Pharmaceutical Chemistry 2020 is a specially designed cluster conference and would lay a platform for the interaction among specialists, directors, professors, faculties, experts and research fellows around the world reputed research institutes, universities and companies, agencies, association & societies. It aims in accelerating the scientific discoveries in the field of Pharma and Chemistry to exchange information on their latest research progress. The organizing committee is gearing up for an exciting and informative event procuring plenary lectures, symposia, workshops on a variety of topics, poster presentations and various sessions for participants from all over the world. All the members of Pharmaceutical Chemistry 2020 organizing committee look forward to meet you at London, UK.
Details of Pharmaceutical Chemistry 2020 in London:
|
Conference Name |
Place |
Date |
|
London, UK |
November 23-24, 2020 |
Why to attend???
With all the scientific people over the world focused on learning about Pharmaceutical Current and Novel trends and advanced strategies in Pharma technology and Chemistry research areas. This is a best globalised opportunity to reach the largest assemblage of participants from the Pharma and Chemistry community. We anticipate participants, renowned speakers, pharmacists/chemist and eminent delegates across the globe attending the conference to share their valuable presentation and galvanize the scientific community. Pharmaceutical Chemistry 2020 is a two day event offering the Exhibition at venue to showcase the new and emerging technologies and Conduct presentations, distribute information, meet with potential scientists, make a splash with new drug developments, and receive fame and recognition. Our services have always met with great achievement in Business Conferencing. World-renowned speakers, the most recent and advanced techniques, developments, and the newest updates are the prominent features of the conference.
Young Scientist Benefits
- Our conferences provide best Platform for your research through oral presentations.
- Share the ideas with both eminent researchers and mentors.
- Young Scientist Award reorganization certificate and memento to the winners
- Young Scientists will get appropriate and timely information by this Forum.
- Platform for collaboration among young researchers for better development
- Award should motivate participants to strive to realize their full potential which could in turn be beneficial to the field as whole.
Deadline for Registrations:
- Till March 28, 2020 – $399
- Till April 28, 2020 – $499
- Till November 20, 2020 – $599
Target Audience:
Eminent Scientific Professionals in Pharma and Chemistry
Faculty (Professors, Associate Professors, Asst. Professors)
Pharma and Chemistry Colleges & Training Institutes
Pharmaceutical and Chemistry Associations and Societies
Pharmaceutical Business Entrepreneurs
Manufacturing Pharmaceutical products Companies
Manufacturing Medical Devices Companies
Pharma and Chemistry Students
PhD Scholars, Graduates and Post Graduates
Directors, CEO’s of Organizations
Association, Association presidents and professionals
Noble laureates in Health Care and Medicine
Bio instruments Professionals
Bio-informatics Professionals
Research Institutes and members
CRO and DATA management Companies
Data Management Companies
Track 1: Pharmaceutical Chemistry Contemporary Topics
Medicinal chemistry is the discovery, design and synthesis of potential new drugs. Pharmaceutical chemistry is the development of these into optimized drugs that can be sold as pharmaceuticals. The discovery and design of drugs requires an understanding of the biology of a disease to find an effective target for the drug, and will often involve computer-based in silico discovery and design of the drug structure. Research in Medicinal and Pharmaceutical Chemistry also includes the synthesis of the designed drugs, the optimization of their structures and action, and the development of methods for producing the drugs on a larger scale; these are challenging tasks which involve working in multidisciplinary teams with other members of the pharmacy research team as well as biologists, mathematicians and engineers.
Related Journals: Journal of Medicinal Chemistry | Current Topics in Medicinal Chemistry | Future Medicinal Chemistry | European Journal of Medicinal Chemistry | Anti-Cancer Agents in Medicinal Chemistry
Track 2: Drug Discovery and Development
Drug discovery is a process which is intended to identify a small synthetic molecule or a large biomolecule for comprehensive evaluation as a potential drug candidate. Broadly, the modern drug discovery process includes identification of disease to be treated and its unmet medical need, selection of a druggable molecular target and its validation, in vitro assay development followed by high throughput screening of compound libraries against the target to identify hits, and hit optimization to generate lead compounds that exhibit adequate potency and selectivity towards the biological target in vitro and which demonstrate efficacy in animal models of disease. Subsequently, the lead compounds are further optimized to improve their efficacy and pharmacokinetics before they advance towards drug development.
Drug development process can be segregated into preclinical and clinical development stages. In preclinical development, toxicological and safety pharmacology studies of the candidate are conducted in order to establish the maximum safe concentrations in animals and determine the adverse effect potential of the drug-in-development. Additionally, studies are conducted to finalize cost-effective processes required for manufacturing the candidate drug as well as deciding on its best formulation. If the candidate exhibits sufficient efficacy and safety in preclinical evaluation, permission is sought from drug regulatory agencies to initiate its clinical development wherein the safety and efficacy of the drug candidate is assessed in pilot and pivotal studies.
Related Journals: Drug Discovery Today | Nature Reviews Drug Discovery | Drug Research | Drug Design, Development and Therapy | Pharmaceutical Development and Technology
Track 3: Drug Designing Methodologies
Design of a novel drug is one of the biggest challenges faced by the pharmaceutical industry. The use of computers accelerates the process of drug design which is a time intensive process, and also reduces the cost of whole process. Computational methods are used in various forms of drug discovery like QSAR, virtual screening and structure-based drug designing methods. Among these, structure based drug design is gaining importance due to rapid growth in structural data (available in RCSB & Nucleic acid Data Bank). This structural data can be used in molecular modeling to design lead molecules based on the structural features of the active site.
Related Journals: Chemical Biology and Drug Design | Journal of Drug Design and Research | Journal of Drug Design and Medicinal Chemistry | Research & Reviews: A Journal of Drug Design & Discovery | Modern Approaches in Drug Designing
Track 4: Clinical Research
Clinical research is the study of health and illness in people. It is the way we learn how to prevent, diagnose and treat illness. Clinical research describes many different elements of scientific investigation. Simply put, it involves human participants and helps translate basic research (done in labs) into new treatments and information to benefit patients. Clinical trials as well as research in epidemiology, physiology and pathophysiology, health services, education, outcomes and mental health can all fall under the clinical research umbrella.
Related Journals: Clinical Trials | Contemporary Clinical Trials | Clinical Medicine & Research | Clinical Case Studies | Journal of Clinical Pathology
Track 5: Natural Products as Leads for New Pharmaceuticals
A review of the use of natural products as starting points in the search for new pharmaceuticals, covering the broad areas of Drugs affecting the central nervous system, Neuromuscular blocking drugs, Anticancer drugs, Marine sources, Antibiotics, Cardiovascular drugs, Antiasthma drugs, Anti-diabetic drugs and Antiparasitic drugs. The review covers more than 100 years of development, from “old” drugs, such as morphine and quinine, to very recent discoveries, such as conotoxin and galantamine, and includes a discussion of the attributes of natural products as leads in the drug discovery process. Between 1990 and 2000, a total of 41 drugs derived from natural products were launched on the market by major pharmaceutical companies. In the chosen examples, the process of drug development is traced from the discovery of the activity of the natural compound, through chemical modification and biological evaluation, to either success or failure as a clinical product, highlighting the very different pathways to innovation that occur in each product.
Related Journals: Pharmacognosy Journal | Journal of Natural Products | Natural Product Research | Chemistry of Natural Compounds | Marine Drugs
Track 6: Pharmaceutical Biotechnology in Drug Development
Pharmaceutical biotechnology is a relatively new and growing field in which the principles of biotechnology are applied to the development of drugs. A majority of therapeutic drugs in the current market are bioformulations, such as antibodies, nucleic acid products and vaccines. Such bioformulations are developed through several stages that include: understanding the principles underlying health and disease; the fundamental molecular mechanisms governing the function of related biomolecules; synthesis and purification of the molecules; determining the product shelf life, stability, toxicity and immunogenicity; drug delivery systems; patenting; and clinical trials.
Related Journals: Current Pharmaceutical Biotechnology | Nature Biotechnology | Journal of Applied Biology & Biotechnology | Journal of Biotechnology | Biotechnology Journal
Track 7: Green Chemistry in Pharma Industry
Green chemistry expresses an area of research developing from scientific discoveries about pollution awareness and it utilizes a set of principles that reduces or eliminates the use or generation of hazardous substances in all steps of particular synthesis or process. Chemists and medicinal scientists can greatly reduce the risk to human health and the environment by following all the valuable principles of green chemistry. The most simple and direct way to apply green chemistry in pharmaceuticals is to utilize eco-friendly, non-hazardous, reproducible and efficient solvents and catalysts in synthesis of drug molecules, drug intermediates and in researches involving synthetic chemistry. Microwave synthesis is also an important tool of green chemistry by being an energy efficient process.
Related Journals: Asian Journal of Green Chemistry | International Journal of Green and Herbal Chemistry | Green Chemistry & Technology Letters | Trends in Green Chemistry | Current Opinion in Green and Sustainable Chemistry
Track 8: Computational Chemistry
Computational chemistry within the pharmaceutical industry has grown into a field that proactively contributes too many aspects of drug design, including target selection and lead identification and optimization. While methodological advancements have been key to this development, organizational developments have been crucial to our success as well. In particular, the interaction between computational and medicinal chemistry and the integration of computational chemistry into the entire drug discovery process have been invaluable.
Related Journals: Computational and Theoretical Chemistry | Journal of Computational Chemistry | Journal of Theoretical and Computational Chemistry | Computational Biology and Chemistry | Journal of Chemical Theory and Computation
Track 9: Organic Pharmaceutical Chemistry
Organic chemistry is one of the branches of chemistry that majorly deals with the study of different molecular structures, compositions, properties and synthesis of different compounds. These compounds under study majorly contain hydrocarbons, carbon and their derivatives. The combination of these compounds in an effort to come up with different medicinal ingredients can be put under synthetic organic chemistry procedures.
The science of pharmaceuticals is a field with disciplines that work together. This discipline usually works together through synthetic organic chemistry to bring out pharmaceutical drug compounds with intense effects. Synthetic organic chemistry and pharmaceutical chemistry are both involved in the manufacture of pharmaceutical drugs.
Related Journals: European Journal of Organic Chemistry | Organic and Biomolecular Chemistry | Organic Process Research & Development | Journal of Organometallic Chemistry | Beilstein Journal of Organic Chemistry
Track 10: Inorganic Chemistry
Inorganic chemistry is the study of all the elements and their compounds except carbon and its compounds. Inorganic chemistry describes the characteristics of substances such as non-living matter and minerals which are found in the earth except the class of organic compounds. Branches of inorganic chemistry include coordination chemistry, bioinorganic chemistry, organometallic compounds and synthetic inorganic chemistry. The distinction between the organic and inorganic are not absolute, and there is much overlap, especially in the organometallic chemistry, which has applications in every aspect of the pharmacy, chemical industry–including catalysis in drug synthesis, pigments, surfactants and agriculture. In short, Inorganic chemistry is the branch of chemistry that deals with inorganic compounds. In other words, it is the chemistry of compounds that do not contain hydrocarbon radicals.
Related Journals: Journal of Inorganic Chemistry | Inorganic Chemistry | European Journal of Inorganic Chemistry | Inorganic Chemistry: An Indian Journal | Russian Journal of Inorganic Chemistry
Track 11: Heterocyclic Chemistry
New advances in synthetic methodologies that allow rapid access to a wide variety of functionalized heterocyclic compounds are of critical importance to the medicinal chemist as it provides the ability to expand the available drug-like chemical space and drive more efficient delivery of drug discovery programs. Furthermore, the development of robust synthetic routes that can readily generate bulk quantities of a desired compound help to accelerate the drug development process. While established synthetic methodologies are commonly utilized during the course of a drug discovery program, the development of innovative heterocyclic syntheses that allow for different bond forming strategies are having a significant impact in the pharmaceutical industry. This review will focus on recent applications of new methodologies in C-H activation, photoredox chemistry, borrowing hydrogen catalysis, multicomponent reactions, regio- and stereoselective syntheses, as well as other new, innovative general syntheses for the formation and functionalization of heterocycles that have helped drive project delivery.
Related Journals: Journal of Heterocyclic Chemistry | Chemistry of Heterocyclic Compounds | American Journal of Heterocyclic Chemistry | International Journal of Heterocyclic Chemistry | Moroccan Journal of Heterocyclic Chemistry
Track 12: Pharmaceutical Formulation
Pharmaceutical formulation is the process of combining various chemical substances with the active drug to form a final medicinal product, which is called a drug mixture or drug formulation. A drug formulation can be given to the patient in various forms like solid, semisolid or liquid. The type of the formulation given depends upon the patient’s age, sex, and health condition and is specific for particular routes of administration.
Related Journals: Research & Reviews: A Journal of Drug Formulation, Development and Production | International Journal of Drug Formulation and Research | Pharmaceutical Development and Technology | Recent Patents on Drug Delivery & Formulation | Drug Development Journals
Track 13: Pharmaceutical Analysis
Pharmaceutical analysis is a branch of practical chemistry that involves a series of process for identification, determination, quantification and purification of a substance, separation of the components of a solution or mixture, or determination of structure of chemical compounds. The substance may be a single compound or a mixture of compounds and it may be in any of the dosage form. The substance used as pharmaceuticals are animals, plants, microorganisms, minerals and various synthetic products.
Related Journals: Journal of Pharmaceutical Analysis | Current Pharmaceutical Analysis | Asian Journal of Pharmaceutical Analysis | International Journal of Pharmaceutical Quality Assurance | Journal of Analytical & Pharmaceutical research
Track 14: Drug Delivery Systems
Drug delivery systems are engineered technologies for the targeted delivery and/or controlled release of therapeutic agents. Drugs have long been used to improve health and extend lives. The practice of drug delivery has changed dramatically in the past few decades and even greater changes are anticipated in the near future. Biomedical engineers have contributed substantially to our understanding of the physiological barriers to efficient drug delivery, such as transport in the circulatory system and drug movement through cells and tissues; they have also contributed to the development several new modes of drug delivery that have entered clinical practice.
Related Journals: Advanced Drug Delivery Reviews | Journal of Controlled Release | Expert Opinion on Drug Delivery | Journal of Drug Targeting | Theranostics
Track 15: Pharmacology
Pharmacology is the study of how a drug affects a biological system and how the body responds to the drug. The discipline encompasses the sources, chemical properties, biological effects and therapeutic uses of drugs. These effects can be therapeutic or toxic, depending on many factors. Pharmacologists are often interested in therapeutics, which focuses on the effects of drugs and other chemical agents that minimize disease, or toxicology, which involves the study of adverse, or toxic, effects of drugs and other chemical agents. Toxicology can refer to both drugs used in the treatment of disease and with chemicals that may be present in household, environmental, or industrial hazards.
Related Journals: Journal of Pharmacy and Pharmacology | Journal of Clinical Pharmacology | British Journal of Pharmacology | British Journal of Clinical Pharmacology | Bangladesh Journal of Pharmacology
Track 16: Toxicology
Toxicology is a field of science that helps us understand the harmful effects that chemicals, substances, or situations, can have on people, animals, and the environment. Some refer to toxicology as the “Science of Safety” because as a field it has evolved from a science focused on studying poisons and adverse effects of chemical exposures, to a science devoted to studying safety. Toxicology uses the power of science to predict what, and how chemicals may cause harm and then shares that information to protect public health.
Related Journals: International Journal of Toxicology | Journal of Medical Toxicology | Journal of Toxicology and Environmental Health | Journal of Applied Toxicology | Journal of Immunotoxicology
In addition to Sessions/Tracks, papers dealing with new areas of Pharma and Chemistry that fit the broad scope and objectives of the Conference are encouraged.
While the global pharmaceutical market is forecast to grow steadily over the next five years, a number of common opportunities and challenges are evident across different markets. Initiatives are being taken to improve access to innovative products, but cost containment remains high on payers’ agendas in all countries, and will contribute to a gradual slowing in annual growth rates over the five-year period.
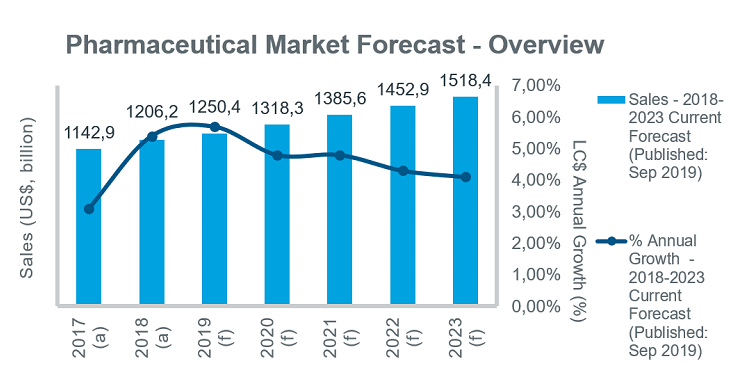
While growth will remain steady in the EU5 markets, key priorities for governments over the forecast period will be price control initiatives and measures to improve patient access. In Germany the launch of new drugs will drive market growth, with at least 30 launches expected in 2019. However, the use of early benefit assessments and unpredictable outcomes of price negotiations remain an issue for manufacturers of innovative drugs. The GSAV law, which came into effect in August 2019, covers a number of different topics, including: provisions for adjusting orphan drug prices; ensuring security of supply in discount contracts between companies and health insurance funds; and measures to increase uptake of biosimilars. France is also introducing measures to improve biosimilar uptake, aiming to achieve 80% penetration rates by 2022.
Growing emphasis will be put on disease prevention and the control of chronic diseases under recent and current policy initiatives, including the new health law adopted in July 2019, which targets a transformation of the healthcare system. In Spain, price controls as a means to curb pharmaceutical spend will be ramped up over the forecast period. In Italy, the long-awaited new pharmaceutical governance strategy (published in December 2018) outlines measures to address issues affecting the underfunded healthcare system and is expected to help contain drug costs while improving patient access to new drugs. In the UK steps have also been taken to expedite patient access to new medicines through initiatives such as the Accelerated Access Collaborative, and reforms to the Cancer Drug Fund. The new voluntary pricing system for branded medicines, effective since January 2019, also aims to accelerate and extend the uptake of new medicines.
Moves to improve access to new medicines in all markets will be increasingly linked with pricing/risk-sharing arrangements. With the uncertainty about Brexit continuing, associations representing the European and British Life Science Industry have warned about the challenges across a range of business areas.
Related Societies and Associations:
United Kingdom
Association of the British Pharmaceutical Industry (ABPI) | UK Clinical Pharmacy Association | Scottish Medicines Consortium (SMC) | Royal Pharmaceutical Society (RPS) | Pharmacists' Defence Association (PDA) | Pharmaceutical Society of Northern Ireland | Pharmaceutical Services Negotiating Committee (PSNC) | National Patient Safety Agency (NPSA) | National Institute for Health and Care Excellence (NICE) | Medicines and Healthcare products Regulatory Agency (MHRA) | General Pharmaceutical Council (GPhC) | Pharmaceutical Society of Ireland | National Pharmacy Association | Royal Society of Chemistry (RSC)
Europe
European Association of Employed Community Pharmacists in Europe (EPhEU) | Pharmaceutical Group of the European Union (PGEU) | Danish Association of Pharmaconomists | Norwegian Pharmacy Association | Belgian Society of Biochemistry and Molecular Biology | Biochemical Society | Danish Chemical Society | The Electrochemical Society | European Association for Chemical and Molecular Sciences | Faraday Society | Federation of European Biochemical Societies | Gesellschaft Deutscher Chemiker (GDCh) | Hungarian Chemical Society | Italian Chemical Society (SCI) | Norwegian Chemical Society | Polish Chemical Society | Royal Netherlands Chemical Society (KNCV) | Société Chimique de France | Society of Chemical Industry (SCI) | Society of Chemical Industry (American Section) | Society of Chemical Industry (American Section) | Society of Cosmetic Chemists | Swedish Chemical Society | World Association of Theoretical and Computational Chemists
America
American Association of Colleges of Pharmacy (AACP) | American Pharmacists Association (APhA) | American Society for Pharmacy Law | American Society of Consultant Pharmacists (ASCP) | American Society of Health-System Pharmacists (ASHP) | Canadian Pharmacists Association | Canadian Society of Hospital Pharmacists | Ontario Pharmacists Association | Alpha Chi Sigma (ΑΧΣ) | American Association for Clinical Chemistry | American Chemical Society | American Crystallographic Association | American Oil Chemists' Society | American Society of Brewing Chemists | American Society for Mass Spectrometry | Association of Analytical Communities (AOAC International) | Association of Greek Chemists | Brazilian Chemical Society | Canadian Society for Chemical Technology (CSCT) | Canadian Society of Clinical Chemists (CSCC) | Chemical Society of Peru
Asia, Africa & Oceania
Pharmaceutical Society of Australia | The Society of Hospital Pharmacists of Australia | Chinese Pharmaceutical Association | The Pharmaceutical Association of Israel | Kuwait Pharmaceutical Association | Pharmaceutical Association of Mauritius | Pharmaceutical Society Of New Zealand | Pakistan Pharmacists Association | Chemical Society Located in Taipei (CSLT) | Chemical Society of Japan (CSJ) | Chemical Society of Pakistan | Chinese-American Chemical Society | Chinese Chemical Society (Beijing) (CCS) | Chinese Chemical Society (Taipei) (CSLT) | Council for Chemical Research (CCR) | Chemical Research Society of India | Indian Chemical Society | International Mass Spectrometry Foundation | International Union of Crystallography | International Union of Pure and Applied Chemistry (IUPAC) | Iranian Chemists Association | Japan Association for International Chemical Information | Journal of the Chemical Society of Pakistan | The Korean Chemical Society
Conference Highlights
- Pharmaceutical Chemistry Contemporary Topics
- Computational Chemistry
- Drug Discovery and Development
- Drug Designing Methodologies
- Natural Products as Leads for New Pharmaceuticals
- Pharmaceutical Biotechnology in Drug Development
- Green Chemistry in Pharma Industry
- Organic Pharmaceutical Chemistry
- Heterocyclic Chemistry
- Pharmaceutical Formulation
- Pharmaceutical Analysis
- Pharmacology
- Drug Delivery Techniques
- Clinical Research
- Toxicology
- Inorganic Chemistry
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | November 23-24, 2020 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Chemical Biology & Pharmaceutical Chemistry
- Research & Reviews: Journal of Chemistry
- Industrial Chemistry: Open Access
Abstracts will be provided with Digital Object Identifier by



