Theme: Exploring the Challenges in Pharmaceutical Chemistry & Process of Drug Discovery for the Future
Pharmaceutical Chemistry 2016
Track 1: Prospective of Pharmaceutical Chemistry
Pharmaceutical Chemistry is that the science handling the composition and preparation of chemical compounds utilized in medical diagnoses and therapies. Medicinal chemistry and pharmaceutical chemistry square measure disciplines at the intersection of chemistry, particularly especially synthetic organic chemistry, and pharmacology and numerous alternative biological specialties, wherever they're committed style, chemical synthesis and development for market of pharmaceutical agents, or bio-active. Pharmaceutical chemistry encompasses drugs design, drug developed, drug synthesis , and also the analysis of drug effectivity (how effective it's in treating a condition) and drug safety. before the nineteenth century, colleges of pharmacy trained pharmacists and physicians the way to prepare medicative remedies from natural organic product or inorganic materials. By discovering and structurally characterizing compounds with medicative activity, chemists square measure ready to style new medicine with increased efficiency and shriveled adverse side effects.
Drug discovery is that the core of pharmaceutical chemistry. The drug discovery method includes all the stages of drug development, from targeting a sickness or medical condition to toxicity studies in animals, or even, by some definitions, testing the drug on human subjects. Typically, conditions that have an effect on a bigger proportion of the population health, receive a lot of attention and a lot of analysis funding. Antiulcer medicine and Diabetic cholesterol- reducing agents square measure presently the therapeutic areas of greatest emphasis. To develop a drug to target a specific disease, researchers try to understand the biological mechanism responsible for that condition. If the biochemical pathways leading up to the disease are understood, scientists attempt to design drugs that will block one or several of the steps of the disease's progress. Alternatively, drugs that boost the body's own defence mechanism may be appropriate.
Related Conferences of Pharmaceutical Chemistry:
Pharmaceutical Summit and Expo, October 08-10, 2015, New Delhi, India; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE ; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey; European Chemistry Congress, June 16-18, 2016 Rome, Italy; 18th SCI/RSC Medicinal Chemistry Symposium, Cambridge, United Kingdom; FMC 2015 Frontiers in Medicinal Chemistry, Antwerp, Belgium; 14th International Conference on the Chemistry of Antibiotics and other Bioactive Compounds, Galveston, United States; The Expanding Toolbox of Medicinal Chemistry: From Chemical Biology to Clinical Applications, Dijon, France; 1st RSC / SCI symposium on Fibrosis Disease: medicinal chemistry progress from biological target to the clinic, Slough, United Kingdom, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
American Association of Pharmaceutical Scientists (AAPS)
Association of the British Pharmaceutical Industry (ABPI)
European Federation for Pharmaceutical Sciences (EUFEPS)
Track 2: Genesis of Novel Drugs:
Computer aided drug design is the foremost elementary goal to predict whether or not or not a given molecule can bind to a target or not. Study in drug design or biomarkers in drug style measures the conformation of the limited molecule and to model conformational changes at intervals the biological target which is able to occur once the limited molecule binds to that. Recent advances in laptop assisted drug style with the use of high performance computing technology and insilco molecular design coding system and tools supply would possibly optimize the parameters for the molecular mechanics calculations and in addition offer an estimation of the electronic properties (electrostatic potential, polarizability, etc.) of the drug molecule that will influence binding affinity at intervals the employment of ultrasound in healthful chemistry. high-resolution 1H-NMR qualitative analysis and X-Ray absorption qualitative analysis ways in which could in addition be accustomed supply semi-quantitative prediction of the binding affinity.
Related Conferences of Genesis of Novel Drug:
4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey; 7th International Conference on Biomarkers & Clinical Research, November 28-30, 2016 Baltimore, USA; ; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; 1st Conference on Recent Trends in Drug Development, Muscat, Oman, Asia ; Functional Analysis & Screening Technologies Congress, Boston, United States; 7th International Conference on Drug Discovery and Therapy, Dubai, United Arab Emirates; Fragment-Based Drug Discovery Conference, San Diego (CA), USA, North America; Phenotypic Approaches in Drug Discovery, London, United Kingdom, Europe, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
American Society of Clinical Oncology (ASCO)
European Federation for Medicinal Chemistry (EFMC)
Track 3: Technologies in Natural Product
Drug discovery leading to robust and viable lead candidates’ remains a difficult scientific task, that is that the transition from a screening hit to a drug candidate, needs experience and knowledge. Natural product and their derivatives are recognized for several years as a supply of therapeutic agents and of structural diversity. However, additionally to their chemical structure diversity and their multifariousness, the event of recent technologies has revolutionized the screening of natural product in discovering new medication. Applying these technologies compensates for the inherent limitations of natural product and offers a novel chance to re-establish natural product as a significant supply for drug discovery. This topic involves the makes an attempt to explain the employment of compounds derived from natural resources as drug candidates, with a spotlight on the success of those resources within the method of finding and discovering new and effective drug compounds, associate approach ordinarily mentioned as “natural product drug discovery”.
Related Conferences of Natural Products:
3rd International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, October 26-28, 2015 Hyderabad, India; Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; ; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey; 1st Conference on Recent Trends in Drug Development, Muscat, Oman, Asia ; Functional Analysis & Screening Technologies Congress, Boston, United States; 7th International Conference on Drug Discovery and Therapy, Dubai, United Arab Emirates; Fragment-Based Drug Discovery Conference, San Diego (CA), USA, North America; Phenotypic Approaches in Drug Discovery, London, United Kingdom, Europe, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Malaysian Natural Product Society (MNPS)
The American Society of Pharmacognosy
The Natural Products Association
Federation of Asian Chemical Societies (FACS)
Track 4: QSAR in Pharmaceutical Chemistry
Quantitative structure–activity relationship models (QSAR models) square measure regression or classification models employed in the chemical and biological sciences and engineering. Like alternative regression models, QSAR regression models relate a collection of "predictor" variables (X) to the efficiency of the response variable (Y), whereas classification QSAR models relate the predictor variables to a categorical price of the response variable. In QSAR modelling, the predictors contains physico-chemical properties or theoretical molecular descriptors of chemicals; the QSAR response-variable may be a biological activity of the chemicals. QSAR models initial summarize a supposed relationship between chemical structures and biological activity in an exceedingly data-set of chemicals. Second, QSAR models predict the activities of recent chemicals.
Related terms embody quantitative structure–property relationships (QSPR) once a property is modelled because the response variable. As associate degree example, biological activity may be expressed quantitatively because the concentration of a substance needed to relinquish an explicit biological response. in addition, once chemical science properties or structures square measure expressed by numbers, one will notice a mathematical relationship, or quantitative structure-activity relationship, between the 2. The mathematical expression, if fastidiously valid will then be wont to predict the modelled response of alternative chemical structures.
Related Conferences of QSAR studies:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; ; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey; 16th Workshop on Quantitative Structure-Activity Relationships, Milan, Italy; 20th Euro QSAR, Understanding Chemical-Biological Interactions, St. Petersburg, Russia; 17th Conference on QSAR in Environmental and Health Sciences, Florida, USA; 19th European Congress on Alternatives to Animal Testing, Linz, Austria; 11th Triennial Congress of the World Association of Theoretical and Computational Chemists, Munich, Germany, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Canadian Society for Chemistry
Computational Chemistry Working Party UK
European Photochemistry Association (EPA)
Track 5: Drug Discovery and Therapy
National Cancer Institute (NCI) provides preclinical cancer research, which includes evaluating the preclinical safety and pharmacokinetics of chemotherapeutics and chemopreventive agents. Many research Laboratories also provides genetic toxicology testing for NCI's chemoprevention program, synthesizes compounds for NCI's carcinogen repository, and manufactures clinical trial materials.
National Cancer Institute (NCI) funded for Tumour Glycome Laboratories. They are the principal component of the trans-NIH Alliance of Glycobiologists for Detection of Cancer and Cancer Risk, which is searching for glycan-based biomarkers for breast, ovarian, lung, prostate and pancreatic cancers and melanoma.
More than 30 years of research has led to numerous drugs on the market or ready for licensing, including:
· Targretin® (bexarotene), a retinoid receptor ligand marketed by Eisai
· Tirapazamine, a hypoxic tissue-selective cytotoxin
· TAS-108, a selective estrogen receptor modulator licensed to Taiho Pharmaceutical
· PDX (pralatrexate), an antimetabolite licensed to Allos Therapeutics
· SR16157, a dual estrone sulfatase / ER antagonist for breast cancer treatment
Related Conferences of Drug discovery Approach in Cancer Drugs:
International Conference and Expo on Molecular & Cancer Biomarkers, September 15-17, 2016 Berlin. Germany; 4th World Congress on Cancer Science & Therapy, October 20-22, 2014 Chicago, USA; Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; International Conference on Biomarkers & Clinical Research, November 28-30, 2016 Baltimore, USA; RICT 2015 - Drug Discovery and Selection, Avignon, France, Europe; Optimizing Small Molecules for Tomorrow's Therapeutics Conference, San Diego (CA), USA, North America; Fragment-Based Drug Discovery Conference, San Diego (CA), USA, North America; Phenotypic Approaches in Drug Discovery, London, United Kingdom, Europe; 16th Conference on Alzheimer's Drug Discovery, Jersey City, United States; VI Inter-American Oncology Conference 'Current Status and Future of Anti-Cancer Targeted Therapies', Buenos Aires, Argentina; Technologies in Drug Discovery Summit Europe, Berlin, Germany; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Slovenian Pharmaceutical Society
Track 6: Drug Design and Drug Delivery
Drug design, typically cited as rational drug style or just rational style, is that the ingenious method of finding new medications supported the information of a biological target. The drug is most typically associate organic tiny molecule that activates or inhibits the operate of a biomolecule like a supermolecule, that successively leads to a therapeutic profit to the patient. within the most elementary sense, drug style involves the planning of molecules that square measure complementary in form and charge to the biomolecular target with that they move and so can bind to that. Drug style oft however not essentially depends on pc modeling techniques. this sort of modeling is usually cited as computer-aided drug design. Finally, drug style that depends on the information of the three-dimensional structure of the biomolecular target is thought as structure-based drug style. additionally to tiny molecules, biopharmaceuticals and particularly therapeutic associatetibodies square measure an more and more vital category of medication and machine ways for up the affinity, property, and stability of these protein-based therapeutics have also been developed..
The phrase "drug design" is to some extent a name. A a lot of correct term is matter style (i.e., style of a molecule that may bind tightly to its target). though style techniques for prediction of binding affinity square measure moderately triple-crown, there square measure several different properties, like bioavailability, metabolic half-life, side effects, etc., that first should be optimized before a matter will become a secure and efficacious drug. These different characteristics square measure typically tough to predict rational style techniques. yet, attributable to high attrition rates, particularly throughout clinical phases of drug development, a lot of attention is being targeted early within the drug design method on choosing candidate medication whose chemical science properties square measure foretold to end in fewer complications throughout development associated thence a lot of doubtless to guide to an approved, marketed drug. what is more, in vitro experiments complemented with computation ways square measure {increasingly|progressively|more and a lot of} utilized in early drug discovery to pick compounds with more favorable ADME (absorption, distribution, metabolism, and excretion) and toxicological science profiles.
Related Conferences of Drug Design and Drug Delivery:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey ; Structure Based Drug Design Conference 2016, Carlsbad (CA), USA, North America; Recent Developments in Medical Biotechnology and Structure Based Drug Designing’, Guwahati, India; Drug Discovery & Therapy World Congress 2016, Boston, MA, USA; 4th Annual Discovery Chemistry & Drug Design Congress 2016, Berlin, Germany; 17th International Conference on Computational Biology and Drug Design, Paris, France; 10th Drug Design & Medicinal Chemistry Conference 2016, Berlin, Germany, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Japanese Society of Drug Delivery Systems
Society for Biomaterials (SFB)
Inhalation Drug Delivery Association
Track 7: Molecular Stereochemistry
Stereochemistry is that the study of the static and dynamic aspects of the three-dimensional shapes of molecules. It has long provided a foundation for understanding organic structure and reactivity. At constant time, stereochemistry constitutes associate degree in and of itself fascinating analysis field in its claim. several chemists realize this space of study fascinating due merely to the aesthetic beauty related to use of chemical structures, and also the intriguing ability to combine the fields of geometry, topology, and chemistry within the study of three-dimensional shapes. additionally, there square measure extraordinarily necessary sensible ramifications of stereochemistry. Nature is inherently chiral as a result of the building blocks of life (-amino acids, nucleotides, and sugars) square measure chiral and seem in nature in enantiomerically pure forms. Hence, any substances created by man to move with or modify nature square measure interacting with a chiral environment. this is often a very important issue for bioorganic chemists, and a sensible issue for pharmaceutical chemists. The Food and Drug Administration (FDA) currently needs that medicine be created in enantiomerically pure forms, or that rigorous tests be performed to confirm that each enantiomers square measure safe.
In addition, stereochemistry is very relevant to unnatural systems. As we'll describe herein, the properties of synthetic polymers square measure extraordinarily dependent upon the stereochemistry of the continuation units. Finally, the study of stereochemistry may be wont to probe reaction mechanisms, and that we can explore the stereochemical outcome of chemical reactions throughout the chapters in elements II and III of this text. Hence, understanding stereochemistry is critical for many fields of chemistry, creating this chapter one amongst preponderant importance. All introductory chemical science courses teach the basics of stereoisomerism, and that we can solely in short review that data here. we have a tendency to conjointly take a rather a lot of trendy viewpoint, accenting newer nomenclature and ideas. The goal is for the scholar to realize a elementary understanding of the essential principles of stereochemistry and also the associated nomenclature, so to gift a number of the fashionable issues and analysis topics during this space.
Related Conferences of Molecular Stereochemistry:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; European Chemistry Congress, June 16-18, 2016 Rome, Italy;; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; ; International Conference and Exhibition on Materials Chemistry, March 31-April 01, 2016 Valencia, Spain; 2nd International Conference on Past and Present Research Systems of Green Chemistry, September 14-16, 2015 Orlando, USA; 50th EUCHEM Conference on Stereochemistry (Bürgenstock Conference), Brunnen, Switzerland; 18th IUPAC International Symposia on Organometallic Chemistry Directed Towards Organic Synthesis (OMCOS 18), Barcelona, Spain, Europe; 44th National Organic Chemistry Symposium, USA, North America; Anatolian Conference on Synthetic Organic Chemistry, Antalya, Turkey, Europe/Asia; 24th Symposium : Synthesis in Organic Synthesis 20 - 23 July 2015, Churchill College, Cambridge; 5th Conference on Frontiers in Organic Synthesis Technology, Budapest, Hungary, Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
American Psychiatric Association
Track 8: Advanced Organic Chemistry
Organic reactions square measure chemical reactions involving organic compounds. the fundamental chemistry reaction sorts square measure addition reactions, elimination reactions, substitution reactions, pericyclic reactions, arranging reactions, chemistry reactions and reaction reactions. In organic synthesis, organic reactions square measure employed in the development of recent organic molecules. the assembly of the many synthetic chemicals like medicine, plastics, food additives, materials depend upon organic reactions. There is no limit to the quantity of doable organic reactions and mechanisms. However, sure general patterns square measure determined that may be accustomed describe several common or helpful reactions. every reaction features a stepwise reaction mechanism that explains however it happens, though this elaborate description of steps isn't invariably clear from a listing of reactants alone. Synthetic organic reactions will be organized into many basic sorts. Some reactions match into over one class. for instance, some substitution reactions follow an addition-elimination pathway.
In general the stepwise progression of reaction mechanisms will be diagrammatical victimization arrow pushing techniques during which incurved arrows square measure accustomed track the movement of electrons as starting materials transition to intermediates and products.
Related Conferences of Organic Chemistry and Reaction Mechanisms:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; European Chemistry Congress, June 16-18, 2016 Rome, Italy; 2nd International Conference on Past and Present Research Systems of Green Chemistry, September 14-16, 2015 Orlando, USA; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; 18th IUPAC International Symposia on Organometallic Chemistry Directed Towards Organic Synthesis (OMCOS 18), Barcelona, Spain, Europe; 44th National Organic Chemistry Symposium, USA, North America; Anatolian Conference on Synthetic Organic Chemistry, Antalya, Turkey, Europe/Asia; 24th Symposium : Synthesis in Organic Synthesis 20 - 23 July 2015, Churchill College, Cambridge; 5th Conference on Frontiers in Organic Synthesis Technology, Budapest, Hungary; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Society of Synthetic Organic Chemistry, Japan
The American Chemical Society (ACS)
Track 9: Heterocyclic Chemistry Compounds
Heterocyclic compound or ring structure may be a cyclic compound that has atoms of a minimum of two completely different components as members of its ring(s). Heterocyclic chemistry is that the branch of chemistry addressing the synthesis, properties and applications of those heterocycles. In distinction, the rings of isocyclic compounds consist entirely of atoms of constant part.
Although heterocyclic compounds could also be inorganic, most contain a minimum of one carbon. Whereas atoms that square measure neither carbon nor hydrogen are normally referred to in organic chemistry as heteroatoms, this can be typically compared to the all-carbon backbone. However this doesn't stop a compound like borazine (which has no carbon atoms) from being tagged "heterocyclic". IUPAC recommends the Hantzsch-Widman language for naming heterocyclic compounds.
Related Conferences of Heterocyclic Chemistry:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; Pharma Middle East, November 02-04, 2015 Dubai, UAE; 2nd International Conference on Past and Present Research Systems of Green Chemistry, September 14-16, 2015 Orlando, USA; European Chemistry Congress, June 16-18, 2016 Rome, Italy; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; Trans Mediterranean Colloquium on Heterocyclic Chemistry, Antalya, Turkey, Europe/Asia; International Symposium on Synthesis and Catalysis 2015, Evora, Portugal, Europe; 25th International Society of Heterocyclic Chemistry Congress, Santa Barbara (CA), USA, North America; 13th Conference on Pure and Applied Heterocyclic Chemistry, Hurghada, Egypt, Africa; 2nd International Symposium on Clinical Pharmacology of Antimalarial Agents, Accra, Ghana; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
International Society of Heterocyclic Chemistry (ISHC)
Canadian Society for Chemistry
Track 10: Pharmaceutical Drug Impurities
Impurities can be closely related to the product that is formed during the synthesis of a bulk drug or it can be decomposition product formed during the storage of the drug. The International Conference on Harmonization (ICH) has published guidelines on impurities in new drug substances, products and residual solvents. According to ICH guidelines, an impurity should not exceed 0.1 % and total impurity should not exceed 1.0 % in manufacturing each batch of the drug. Impurities present in excess of 0.1 % should be identified and quantified by sufficiently selective methods. The process of identification and quantification of impurities is known as impurity profiling. The procedure of impurity profiling begins with the detection of impurities using thin layer chromatography, high-performance liquid chromatography or gas chromatography. The presence of impurities in bulk drug can be identified by using the impurity reference standard, which includes the products of predictable side reactions or degradation products. If the retention time of both (impurities present in the bulk drug and impurity reference standard) match, then the impurities present will be easily identified. In case of unsuccessful identification, an analytical method, either LC/MS or GC/MS, is used. Based on MS information, the structure of the impurity will be proposed. The important step in impurity profiling is the synthesis of the material (impurity standard) with the proposed structure. Retention and spectral matching of the synthesized material with the impurity present in the bulk drug are useful for the analytical method development and method validation. It is essential to know the structure of impurities present in the bulk drug in order to alter the drug reaction conditions and to reduce the quantity of impurity to an acceptable level.
Related Conferences of Impurity Profile & its Significance:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; Pharma Middle East, November 02-04, 2015 Dubai, UAE; European Chemistry Congress, June 16-18, 2016 Rome, Italy; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; ; International Conference and Exhibition on Materials Chemistry, March 31-April 01, 2016 Valencia, Spain; Trans Mediterranean Colloquium on Heterocyclic Chemistry, Antalya, Turkey, Europe/Asia; International Symposium on Synthesis and Catalysis 2015, Evora, Portugal, Europe; 25th International Society of Heterocyclic Chemistry Congress, Santa Barbara (CA), USA, North America; 13th Conference on Pure and Applied Heterocyclic Chemistry, Hurghada, Egypt, Africa; 2nd International Symposium on Clinical Pharmacology of Antimalarial Agents, Accra, Ghana; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Japan Society for Analytical Chemistry
Israel Analytical Chemistry Society
International Union of Crystallography
Spanish Society of Chromatography
Pharmacological Studies explores the molecular mechanisms by which drugs cause biological effects. In the broadest sense, pharmacology is the study of how chemical agents, both natural and synthetic (i.e., drugs) affect biological systems. This encompasses investigation of the derivation, chemical properties, physiological and behavioral effects, mechanisms of action, biological transformations, and the therapeutic and non-therapeutic uses of drugs. Pharmacological studies can determine the effects of chemical agents upon subcellular, systemic, physiological or behavioral processes; focus on the treatment and prevention of diseases; or deal with the potential hazards of pesticides and herbicides.
Pharmacology is often described as a bridge science because it incorporates knowledge and skills from a number of basic science disciplines including physiology, biochemistry and cell and molecular biology. Pharmacologists are able to 'translate' such knowledge into the rational development of therapeutics. As a result of their multidisciplinary training, pharmacologists are able to offer a unique perspective in solving drug-, hormone- and chemical-related problems.
Pharmacological Research provides a rapid information exchange medium for specialists within the discipline of pharmacology. The journal publishes papers on basic, translational and clinical research in pharmacology, including clinical trials; it is proud of its rapid publication of accepted papers that comprises a dedicated fast acceptance and publication track for high profile articles.
Related Conferences of Pharmacological Studies:
7th Bioavailability & Bioequivalence: BA/BE Studies Summit August 29-31, 2016 Atlanta, USA; Brazil Pharma Expo August 29-31, 2016 Sao Paulo, Brazil; 6th Pharmaceutical Regulatory Affairs and IPR Conference September 12-14, 2016 San Antonio, USA; 4th Neuropharmacology Conference September 15-17, 2016 San Antonio, USA; 7th European Congress of Pharmacology by the Federation of European Pharmacological Societies and the Turkish Pharmacological Society, June 26 - 30, 2016, Istanbul, Turkey; 29th Congress of the European College of Neuropsychopharmacology(ECNP), September 11 - 20, 2016, Vienna, Austria; Pharmacology 2016 sponsored by the British Pharmacological Society, December 13 - 15, 2016, London, United Kingdom; CPPM 2016 : International Conference on Pharmacology and Pharmaceutical Medicine, Feb 11-12, 2016 Kuala Lumpur, Malaysia; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
American Academy of Veterinary Pharmacology and Therapeutics (AAVPT)
Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT)
Brazilian Society of Pharmacology and Experimental Therapeutics
British Pharmacological Society (BPS)
Track 12: Green Chemistry
Preventing environment pollution is a crucial issue within the current world than cleansing up the atmosphere. By tradition, chemists have designed product that ar effective and economical. They need not thought of the waste generated throughout process of the new chemicals and product and its toxicity. Inventing any new product that are of concern to North American country or ecosystems ultimately goes into the environment at the top of the lifetime of these substances, resulting in accumulation, degradation and toxicity. Hence, it's invariably higher to settle on the process protocols that generate minimum Industrial waste and finished product with few chemicals usage and synthesis steps. Our science builds on nice achievements by chemicals and chemists. It’s been quite a hundred and fifty years that we've got been finding the way to create new molecules. and therefore the modern chemistry with its inventions has improved lots our lives providing an out of this world array of product in each field (consumer product, cloths and electronics) and in the main save lives on this earth with new medicines.
In nature, green chemistry is cleaner, cheaper and smarter and it prevents pollution at molecular level. By definition, it's a philosophy of chemical analysis and engineering that encourages the design of products and processes that minimize the employment and generation of unsafe substances. In ancient chemistry, we have a tendency to use lots of abundance of beginning material to urge the ultimate product and discard most of it as waste. The theme of green chemistry is to maximise the incorporation of the beginning materials or reagents into the ultimate product and conjointly to develop a way that synthesizes a required product by generating basic building blocks (at molecular level), instead of by breaking down a bigger starting material. for creating most of the product (consumer product, cloths, cosmetics and electronics) and chemicals, varied chemicals ar mixed along in larger quantities that ar probably toxic liquids and solvents to urge a final product. and therefore the prescribed drugs also are created within the same fashion to urge a finished chemical product. Hence, most of the waste generated within the chemical industries is solvent connected (80-85%). Now, the industry is historically viewed a lot of as a cause than an answer to pollution, although chemistry offers distinctive solutions within the space of waste prevention.
Related Conferences of Green Chemistry:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; Pharma Middle East, November 02-04, 2015 Dubai, UAE; 2nd International Conference on Past and Present Research Systems of Green Chemistry, September 14-16, 2015 Orlando, USA; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; International Conference and Exhibition on Materials Chemistry, March 31-April 01, 2016 Valencia, Spain; European Chemistry Congress, June 16-18, 2016 Rome, Italy; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; Health Economics for Pharmaceutical Personnel, London, United Kingdom; 2015 Michigan Green Chemistry and Engineering Conference, Ann Arbour , Michigan, USA; Green and Sustainable Chemistry Conference, Berlin, Germany; Flow Chemistry Congress 2015, San Diego, California, USA; International Chemical Congress of Pacific Basin Societies , Honolulu, Hawaii, USA; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
The Phytochemical Society of Europe
The Phytochemical Society of North America
Phytochemical Society of North America
Track 13: Flow Chemistry
Scale-up of any pharmaceutical producing method entails a adept combination of art expertise, science and engineering. Combination of many well-established tools like statistical methods (such as style of Experiments (DOE)), PAT method watching tools, and quantitative modelling tools (such as CFD, DEM, PBE, etc.) could give some blessings over the normal ways of method scale-up within the pharmaceutical sector. Advancement during this direction might facilitate to cause a paradigm shift from “Quality by Testing” to “Rational method Design” inside the spirit of the QbD initiative. Some open queries embody however don't seem to be restricted to: however varied tools said, if used separately, will be applied to or sustained for development process scale-up; however the pharmaceutical sector ought to develop a additional innovative use of the present tools. during this session, we tend to ask for papers that facilitate to answer these 2 queries. Papers that specialise in the employment of quantitative tools (e.g. DOE, PAT, modelling, and theory) toward scaling-up pharmaceutical processes in pharmaceutical formulations and linking to QbD implementation within the pharmaceutical sector area unit significantly inspired. The session can give a forum for open exchange of concepts or innovative use of tools for self-made scale-up of the pharmaceutical processes. Papers regarding all areas of pharmaceutical scale-up, from small molecules to biologics, from drug substances to drug products, are being considered.
Related Conferences of Flow Chemistry:
Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; Pharma Middle East, November 02-04, 2015 Dubai, UAE; 3rd International Conference on Past and Present Research Systems of Green Chemistry, September 19-21, 2016 Las Vegas, USA; International Conference and Exhibition on Materials Chemistry, March 31-April 01, 2016 Valencia, Spain; European Chemistry Congress, June 16-18, 2016 Rome, Italy; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE; 2nd EFMC Young Medicinal Chemist Symposium (EFMC-YMCS 2015), Antwerp, Belgium, Europe; 32nd SCI Process Development Symposium, Cambridge, United Kingdom, Europe; International Congress of Young Chemists - YoungChem2015, Cracow, Poland, Europe; 4th International Conference on Process Engineering and Advanced Materials, Kuala Lumpur, Malaysia, Asia; Process Scale-Up in the Pharmaceutical Industry, Cologne, Germany; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Track 14: Advance Analytical Techniques
Analytical chemistry is that the study of the separation, identification, and quantification of the chemical elements of natural and artificial materials. Qualitative analysis offers a sign of the identity of the chemical species within the sample, and measurement determines the quantity of sure elements within the substance. The separation techniques of elements is commonly performed before analysis.
Analytical strategies may be separated into classical and instrumental. Classical strategies (also referred to as wet chemistry methods) use separations like precipitation, extraction, and distillation and analysis by color, odor, or freezing point. Classical measurement is achieved by mensuration of weight or volume. Instrumental methods use an apparatus to measure physical quantities of the analyte such as light absorption, fluorescence, or conductivity. The separation of materials is accomplished mistreatment action, natural action or field flow fractionation strategies.
Analytical chemistry is additionally centered on enhancements in experimental style, chemometrics, and therefore the creation of latest mensuration tools to produce higher chemical information. Analytical chemistry has applications in forensics, bio analysis, clinical analysis, environmental analysis, and materials analysis.
Related Conferences of Analytical Techniques
World Congress on Chromatography, September 10-12, 2016 Amsterdam, Netherlands; International Conference and Exhibition on Advances in HPLC and Chromatography Techniques, March 17-18, 2016 London, UK; Pharmaceutical Summit and Expo, October 08-10, 2015 New Delhi, India; 2nd International Conference and Expo on Separation Techniques, October 31- November 02,2016 Valencia, Spain; 7th International Conference and Exhibition on Analytical & Bioanalytical Techniques Sept 29-Oct 01, 2016 Miami, USA; PharmaNMR — Application of NMR Spectroscopy in Pharmaceutical Industry, Rovinj, St. Andrew Island, Croatia; HEMS Workshop — Harsh-Environment Mass Spectrometry Workshop, Baltimore, MD, United States; Validation & Transfer of Methods for Pharmaceutical Analysis, Berlin, Germany; Instrumental Methods of Analysis-Modern Trends and Applications (IMA) 2015, Kalamata, Greece; Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy Pittcon 2016, Atlanta, GA, United States; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Japan Society for Analytical Chemistry
Israel Analytical Chemistry Society
International Union of Crystallography
Spanish Society of Chromatography
Track 15: Pharmaceutical Market
Pharmaceutical Chemistry promoting permits a pharmaceutical company or the selected representative to speak with all persons concerned within the promoting and administration of the pharmaceutical product, as well as the physician, the patient and also the pharmacist. A way of implementing a controlled market includes providing tips, to traders, concerning an award related to initial entity-controlled securities that square measure related to a particular project from a minimum of one in all a plurality of an entity's comes. Planning tools guide promoting professionals through steps of acting a scenario health assessment, distinctive opportunities, developing growth strategies, developing growth techniques, and developing measurements. The planning tools square measure personalised to the answers given by promoting professionals and new tools or changed tools will be simply enforced. These tools will be provided during a complete manner, as a part of a network, and as a part of a cooperative atmosphere The planning tools ideally type a part of a complete promoting investment manager which incorporates a market research management resolution, digital quality management, and hooks to existing systems, like client relationship management, finance, producing, and data technology. The promoting management resolution adopts best practices and includes collaboration, project management, campaign management, and analytics, and measure units.
Related Conferences of Pharmaceutical Market:
Pharmaceutical Summit and Expo, October 08-10, 2015, New Delhi, India; 4th International Conference on Medicinal Chemistry & Computer Aided Drug Designing, November 02-04, 2015 Atlanta, USA; International Conference and Expo on Industrial Pharmacy, April 28-29, 2016 Dubai, UAE ; 5th International Conference on Computational Systems Biology; August 22-23, 2016 Philadelphia, USA; 2nd International Conference and Expo on Drug Discovery & Designing, October 24-26, 2016 Istanbul, Turkey; European Chemistry Congress, June 16-18, 2016 Rome, Italy; 18th SCI/RSC Medicinal Chemistry Symposium, Cambridge, United Kingdom; FMC 2015 Frontiers in Medicinal Chemistry, Antwerp, Belgium; 14th International Conference on the Chemistry of Antibiotics and other Bioactive Compounds, Galveston, United States; The Expanding Toolbox of Medicinal Chemistry: From Chemical Biology to Clinical Applications, Dijon, France; 1st RSC / SCI symposium on Fibrosis Disease: medicinal chemistry progress from biological target to the clinic, Slough, United Kingdom; Chemistry Conferences Europe June 16-18, 2016 Rome, Italy
Association of the British Pharmaceutical Industry (ABPI)
Canadian Society for Chemistry
Pharmaceutical Chemistry 2016 organizing committee, OMICS International invites analytical expertise researchers, professors, scientific communities, delegates, students, business professionals and executives to attend the “International Conferences on Pharmaceutical Chemistry” which is to be held during September 05-07, 2016 at Frankfurt, Germany.
Pharmaceutical Chemistry International Conference aim is to collaborate and also to promotes the exchange of ideas, research, mutual enrichment and linkages between research and academic institutions in the Germany, United States and other foreign countries by inviting Nobel Laureates in Pharmaceutical Chemistry, Eminent Scientists in Pharmaceutical Chemistry, Professors in Pharmaceutical Chemistry, CEO’s, Directors and Associated Directors of Pharmaceutical Industries, Deans and Heads of Pharmaceutical Chemistry from Various Institutes and Universities, Young Researchers in Pharmaceutical Chemistry, Research scholars in Pharmaceutical Chemistry, Student’s expertise in the field and provide knowledge on Pharmaceutical Chemistry and unveil the roles and responsibilities if Pharmaceutical Chemist.
In the light of “ Exploring the Challenges in Pharmaceutical Chemistry & Process of Drug Discovery for the Future’’, under this theme, the Conferenceseries aims to provide knowledge on Pharmaceutical Chemistry, Drug Design and various related events to make connections and evaluate opportunities to create an environment that will foster pharmaceutical innovations in organizing Workshops and Symposiums in the field of Pharmaceutical Chemistry. The event Pharmaceutical Chemistry International Conference 2016 will schedule and coordinate meetings with our Editorial Board Members and other experts in various research fields..
Transparency Market Research has published a new report titled “Flow Chemistry Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2014 - 2020.” According to the report, the global flow chemistry market was valued at USD 808.6 M in 2013 and is projected to reach USD 1,526.3 M in 2020, expanding at a CAGR of 9.5% between 2014 and 2020.
OMICS international organises 1000+ global events every year across the globe with support from 1000+ more scientific societies and publishes 700 open access journals which contain over 50000 eminent personalities , reputed scientists as editorial board members.
Market Analysis:
Transparency Market Research has published a new report titled “Flow Chemistry Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2014 - 2020.” According to the report, the global flow chemistry market was valued at US$ 808.6 Mn in 2013 and is projected to reach US$ 1,526.3 Mn in 2020, expanding at a CAGR of 9.5% between 2014 and 2020.
North America has been dominating the demand for flow chemistry over the past few years. The region accounted for over 35% of the global demand for flow chemistry in 2013. Rising demand for flow chemistry in the pharmaceutical industry has been a key factor for the growth of the flow chemistry industry in North America. Asia Pacific followed North America, accounting for the second-largest share in the global flow chemistry market in 2013. Increasing demand for flow chemistry in chemical and pharmaceutical applications is driving the demand for flow chemistry in Asia Pacific. Europe is likely to experience stable growth for flow chemistry in the near future. Market share of Rest of the World is projected to increase in the flow chemistry market in the next few years, led by high demand for flow chemistry in Brazil and Saudi Arabia.
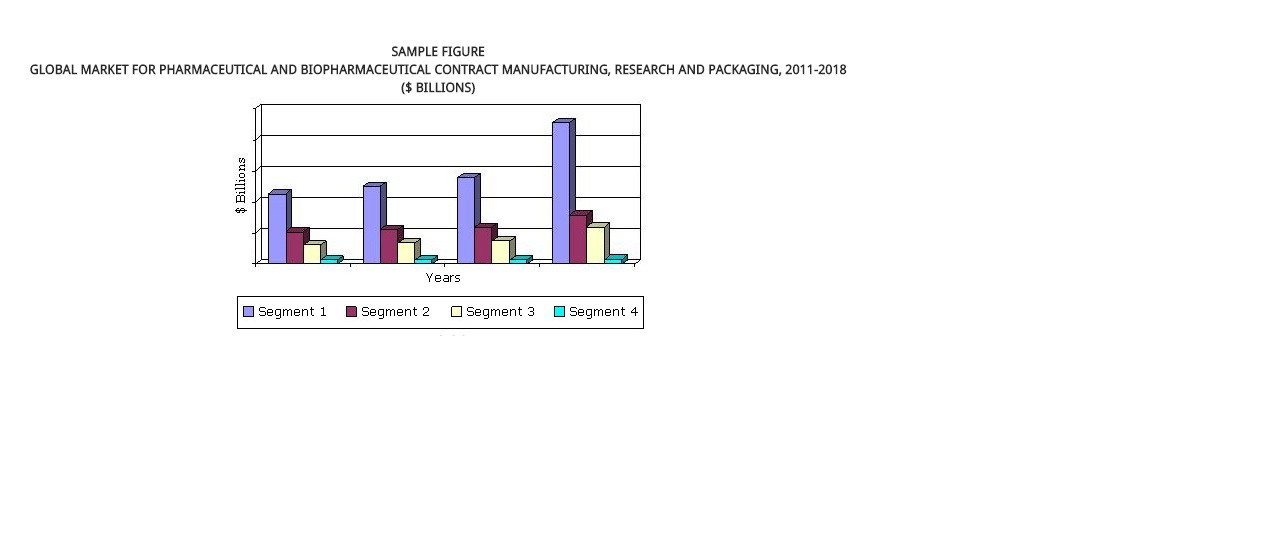
The global market for pharmaceutical and biopharmaceutical contract manufacturing, research and packaging was $219.9 billion in 2012. This market is estimated to reach $242.2 billion in 2013 and $374.8 billion by 2018, a five-year compound annual growth rate (CAGR) of 9.1%.
This report provides:
-
An overview of the global market for contract pharmaceutical manufacturing, research, and packaging, with emphasis on the current and future outlook of the market and a comprehensive analysis of the overall pharmaceutical and biotechnology outsourcing industry.
-
Analyses of global market trends, with data from 2011 and 2012, estimates for 2013, and projections of compound annual growth rates (CAGRs) through 2018.
Conference Highlights
- Drug Discovery and Therapy
- QSAR in Pharmaceutical Chemistry
- Heterocyclic Chemistry Compounds
- Prospectives of Pharmaceutical Chemistry
- Genesis of Novel Drugs
- Technologies in Natural Products
- Drug Design and Drug Delivery
- Molecular Stereochemistry
- Advanced Organic Chemistry
- Green Chemistry
- Modern Analytical Techniques
- Pharmaceutical Drug Impurities
- Flow Chemistry (Pharmaceutical Chemistry)
- Pharmaceutical Market
- Pharmacology Studies
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | September 05-07, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Modern Chemistry and Applications
- Industrial Chemistry: Open Access
- Pharmaceutical Analytical Chemistry: Open Access
Abstracts will be provided with Digital Object Identifier by










































