Yusuf M. Al-Hiari
The University of Jordan, Jordan
Title: Novel Potential Antihyperlipidaemic Agents Derived From Heterocyclic-2-carboxamides
Biography
Biography: Yusuf M. Al-Hiari
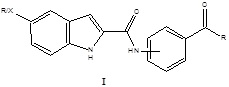
Abstract
More than 5 heterocyclic systems were revealed with potential hypolipidemic activity by our group such as indole, pyrrole, furan, benzofuran and pyridine. All derivatives prepared holds carboxamide attached to highly lipophilic substituents such as benzophenones and antharaquinones. This presentation aims to prepare new 5-substituted indole-1H-indole-2-carboxamide derivatives I as a model and to investigate their hypolipidemic activity in-vivo using Triton WR-1339-induced hyperlipidemic rats as animal model. The novel compounds involve 5-Fluoro-1H-indole-2-carboxamide derivatives of benzophenones1 (series A; 5, 6, 12-15), anthraquinones2 (series B; 17, 19), anilines (series C; 21, 25) and acetophenones (series D; 26-27). The new derivatives represented by compounds 5, 6, 17m and 19 have shown a significant reduction in triglyceride levels (69-90%) compared to hyperlipidemic rats. The analogous 5-Chloro , 5-Bromo and 5-methoxy-1H-indole-2-carboxamide derivatives did not change the pattern of activity with all active derivatives. The elevated plasma triglyceride levels produced by Triton WR-1339 administration were significantly (p < 0.0001) suppressed in bezafibrate (79%), in compound (5) (90%), in compound (6) (83%), in compound (17m) (69%) and (79%) in compound (19) after 12 h in comparison to hyperlipidemic control (HG). At the same time, high-density-lipoprotein cholesterol levels were significantly increased after 12 h of Triton administration (+ 86% p < 0.0001) in compound (5), (+ 53% and +27% p<0.01) in compound (6), BF and (+ 16% and + 37% p<0.05) in compound (17m) and (19) respectively compared to hyperlipidemic control (HG).